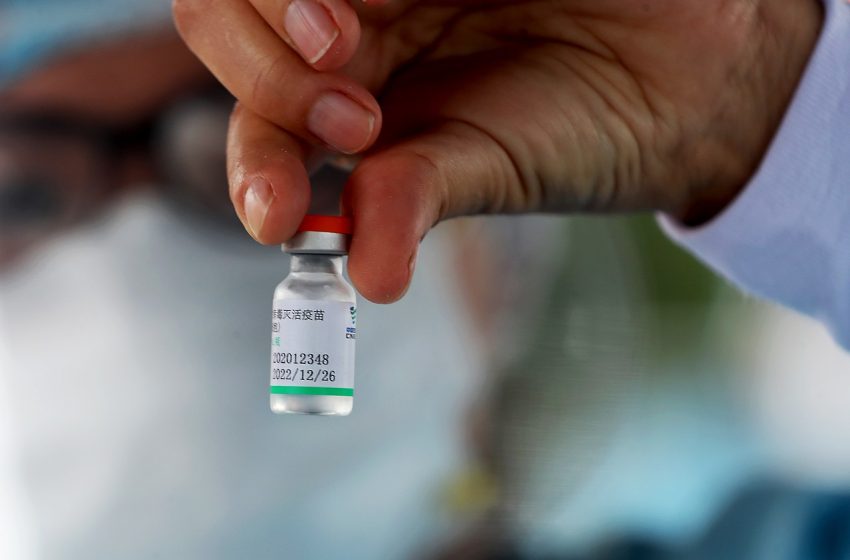
BANGKOK, Sept 21 (TNA) – The Food and Drug Administration has not approved the administration of the COVID-19 vaccine made by Sinopharm for children aged 3 and over on insufficient safety information and urged Biogenetech Co to give more information.
FDA secretary-general Dr. Paisarn Dunkum said Biogenetech which was the approved importer of the Sinopharm vaccine sought permission from the FDA to lower the minimum age of recipients of the Sinopharm vaccine from 18 to 3 years old.
Full story: tna.mcot.net
TNA




















+ There are no comments
Add yours